Chapters
Transcript
[MUSIC PLAYING]
SIVA K. MULPURU: Good evening, everyone. Welcome to the Heart Rhythm Webinar series. Today, we have a special session on genetic heart rhythm conditions. And we have a special guest joining us today. Today, I'm joined by Dr. Asirvatham, my colleagues Dr. DeSimone, Dr. Deshmukh, and Dr. Killu will be joining shortly. Dr. Asirvatham will be introducing our special guest, Dr. Ackerman, today.
SAMUEL J. ASIRVATHAM: Thanks. Thanks, Siva. Thanks, everyone, for joining in. I know this is a difficult time in the places where some of you typically join in. But we have a special session today where the focus will be on something we don't cover when we talk mostly about ablation, but inherited disorders with high risk, especially for sudden death.
We really have the privilege today to have Dr. Michael Ackerman, our colleague here at Mayo, and internationally the go-to person, not only in the ground up understanding of these disorders, original description of the disorders, but most importantly, the day-to-day clinical care of these very difficult questions that these patients and families have when we try to counsel them.
We did have a few questions sent in for us already, and feel free to add further questions. We had also patient case that brings out many of the difficulties where we really need an expert opinion on, that Dr. Ackerman will help in discussing for us. Mike, do you want to say something to our audience? And then we'll have a presentation of that case.
MICHAEL ACKERMAN: Thanks, Sam. It's great to be with all of you. I've been really looking forward to this webinar, and I appreciate how our whole area of genetic cardiology is becoming a true subspecialty, and the aspect of genetic heart rhythm diseases, which as you know, we provide through Mayo Clinic's Wendland Smith Rice Genetic Heart Rhythm Clinic that's dedicated to these families at risk of sudden cardiac death, from both inherited heart rhythm conditions, the cardiac channelopathies, as well as the inherited heart muscle conditions, the cardiomyopathy.
So I'm excited about this case. Looking forward to interacting with all of our colleagues from throughout the globe who've joined us tonight. So thanks for having me Sam, and Siva, and Dr. DeSimone.
SAMUEL J. ASIRVATHAM: So one of the cases that was sent to us was from Dr. John Roshan. John, are you able to present that case?
JOHN ROSHAN: Yes. Thank you.
SAMUEL J. ASIRVATHAM: Thank you very much for joining us. It's a very tough time, and I appreciate all the efforts that you're making there. And hopefully, this learning experience and sharing knowledge will be a little offset for you as well. Do you want to pull up the slides and present, or we can pull it up and present?
JOHN ROSHAN JACOBS: Yeah. Are you able to see my screen?
SAMUEL J. ASIRVATHAM: Yes.
[INTERPOSING VOICES]
SAMUEL J. ASIRVATHAM: And I might stop you from time to time, and then get Mike's opinion as we discuss this case as well.
JOHN ROSHAN JACOBS: Thank you so much for this opportunity. And we, from across the globe, are very grateful to Mayo for improving our discussion and education in the field of EP. Yeah, so we have a 32-year-old male who presented two syncopal episodes.
He's a cycling enthusiast, and his first episode happened when he woke up one morning at 6:30 and decided to go cycling. He usually cycles 35, 40 kilometers, and on this particular day, he woke up, never had anything to eat or drink, straight away took his bike and started cycling. And he says he was cycling at a much faster speed than he normally does. And before he knew it, he passed out and suffered some scrapes and injuries to his arms and knees.
About a month later, when he was actually cycling on a 35 kilometer trail, he decided to push himself further beyond the usual 35 kilometers that he does. And on doing so, he had another episode of syncope, where he again ended up suffering significant lacerations to his knees and elbows.
This gentleman has not had any kind of substance abuse, and there's no history of sudden cardiac death in the family. So he was evaluated for the same, and this was his baseline--
SAMUEL J. ASIRVATHAM: So maybe before we discuss the ECG, clinically this is a scenario that can be tough to know how we develop a differential diagnosis, and young person, otherwise normal, clearly exercise, and then they have syncope. And maybe I'll get Mike to kind of comment on historical points that, to you, are red flags to think more deeply about, and needs careful evaluation. And then I'll ask maybe Dr. Deshmukh to just comment about benign conditions that could present this way as well.
MICHAEL ACKERMAN: Yeah, so thanks, John. Thanks so much for this case. And again, I share my deep concerns with what you're going through in India and throughout the world with our COVID-19 pandemic.
John, I love this case because the challenge, obviously, is when somebody faints without the advantage of an internal loop recorder to give us immediate rhythm spell correlation, you and I and any of us taking these stories, we're trying to envision in our mind, was this, as I view it, a normal sinus rhythm faint, or an arrhythmic faint? And I think when we're young, all of us, by virtue of being young, we have the right to faint [INAUDIBLE] right to faint normally. And in fact, 25% of all of us will have fainted at least once before our 32nd birthday.
We teach, don't we, that exercise-triggered, or exercise-associated, or exercise-related fainting is a dangerous faint until proven otherwise. And so I'm sure you did a full evaluation, as we should. But I think what we fail to teach in that messaging is that even though that's a true statement, we do not have to force a diagnosis of a sudden cardiac death predisposing heart condition on an exertional fainter, because the pre-test probability that an exercise-related fainter has an important life threatening disease is actually only about a 35% chance.
In other words, the vast majority of exercise-related fainting is actually what we'll hear from probably, is just normal vasovagal fainting that occurred during exercise. And I think while we get this out as a warning sign, and we should, I think we sometimes swing the pendulum in the other direction, where we try to force a diagnosis of some sort of channelopathy, some sort of cardiomyopathy, some sort of electropathy on it.
And so Sam, for me, a couple of things that John said in the story that says, yes, it was exercise related, but, and moves me away from the danger faint is, you described how he was doing his exercise, the when he was doing it. The first morning, no nutrition, extreme workout cycling.
What I don't know was, did the faint occur going up a hill, up an ascent, or down a hill? That actually matters, because if this was going up a steep hill in that setting, this would almost for sure be vasovagal syncope without even doing any further workout, without even though it happened during exercise. And so I'm bothered by the setting of the mechanism of faint, in a reassuring way, rather than hearing things that make me more, I don't know, antsy, that this is going to be channelopathic.
And why this is so important, Sam and the colleagues, and I'd love for you to jump in, is if my phenotypic index of suspicion is not tilted towards channelopathic syncope or cardiomyopathy syncope, but tilted towards maybe vanilla syncope while cycling, and I start ordering all those tests, I run a tremendous risk of overinterpreting the findings.
SAMUEL J. ASIRVATHAM: Yeah, I think it's a very common question that comes up, and especially adult electrophysiologists are not aware of the syndrome of neurocardiogenic syncope with effort. It's kind of counterintuitive, but really, the mechanism for standing too long and getting neurocardiogenic syndrome, and extreme effort and getting neurocardiogenic syndrome are very similar. It's that hyperadrenergic phase that comes just before.
Some of the things I use in the history are, did they feel palpitations before they went down? Or did they feel like drained of energy? And if it's someone who's going really fast, going up something, holding their breath, that little extra oomph at the end of an exercise, but then felt energy go down and then they lose consciousness, that often winds up being a neurocardiogenic type. Whereas, if they felt palpitations, dizziness, and then kind of lose recollection before going down, I get a little bit more worried.
Great. You were showing us the ECG, John?
JOHN ROSHAN JACOBS: Yeah, so thank you so much. And so this gentleman was actually cycling on level ground when he had the syncope. He never perceived any palpitations before the episode. But at the same time, he didn't say that he felt drained. It sort of came up on him all of a sudden.
SAMUEL J. ASIRVATHAM: OK.
JOHN ROSHAN JACOBS: So moving on from there, this was his baseline supine EKG, which looking at, we never really saw anything untoward on this ECG. And in the center where he was initially evaluated, he underwent a treadmill test. And as part of the treadmill, when they put him into the standing position, they noticed that his QTc and prolonged quite significantly.
MICHAEL ACKERMAN: John, if I could interject before you show this, because I think you made a really important point about that first ECG. You said it really didn't show anything. I would just say it actually shows something incredibly important. Because you mentioned that you weren't sort of reassured by the story, that it sounded normal fainting, it wasn't a sense of maybe the team was tilted towards a worrisome mechanism for the faint, right?
And then when we think of worrisome fainting in the channelopathies, the first things we think of in exertional syncope are long QT syndrome and catecholaminergic polymorphic ventricular tachycardia, or CPVT. If this was a cardiomyopathic arrhythmogenic faint, probably the first entity we would think about in this entity would be ARVC, or now more collectively called arrhythmogenic cardiomyopathy.
So if we're working towards pursuing those, this ECG almost by itself completely rules out long QT syndrome. Because if that were a QT mediated faint, there's no way his QTc could be this normal at rest. So we talk about normal, concealed long QT syndrome-- and it's true, you can have electrocardiographically concealed long QT syndrome-- but those individuals almost never, not never, but almost never have exertional syncope.
So you are already with this ECG saying, of my three heritable things, I almost have removed long QT syndrome, right? And then, that's when you have to be even more careful when you then do a provocative maneuver, like the standing position QT reactivity so that you don't potentially bite on a finding and an overinterpret what I call, and I'll tell you why, the Sammy stretch. And it's not for Dr. Samuel Asirvatham.
But I'll let you go on and show the stress reaction. But I think this ECG is actually really important, because it would tilt you far away from congenital long QT syndrome.
JOHN ROSHAN JACOBS: So this gentleman was evaluated in another hospital at that point of time, but they subjected him to a treadmill. And when they did so, they noticed that on standing, his QTc had prolonged to 512. So this got them very alarmed. However, all through the treadmill, during the exercise as well as the recovery, the QTc remained absolutely normal.
When he came to our center, we repeated the treadmill, and we never saw any prolongation of his QTc. It remained the same all through. And we also looked at a QTc on the Holter, and never found any abnormal values.
SAMUEL J. ASIRVATHAM: So maybe, Mike, I'll just ask you to comment two questions that came in. Just how do we handle the U wave when measuring the QT interval? And second is just the role of exercise testing in patients that you see and you're evaluating for long QT syndrome. Maybe not a syncope patient, but maybe familial or something else.
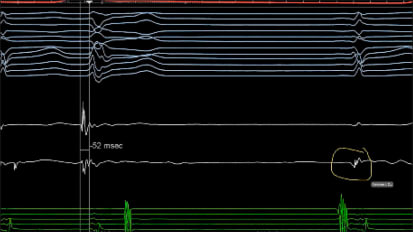
MICHAEL ACKERMAN: So I think first for here, the ECG, there isn't the U wave dilemma on this one and on the stress test. But normally, and I think of the great Peter Schwartz, dear friend of mine, who said, I don't measure the QT interval, I look at it. And so there's the length of repolarization, the QTc, and then there's also the look of repolarization, the T wave morphology and the TU morphology.
And if I think the U wave is a U wave, I exclude it, and I sensor it down the downslope of the T wave and take it at the isoelectric segment there. If I think it's actually a pathologic T wave, and so it's a T wave, a bifed T wave, that doesn't look like normal U wave physiology, and it may be, Sam, you can demonstrate in picture what I'm saying in words, then I would not call it a U wave. I would call it a pathologic T wave and measure it.
SAMUEL J. ASIRVATHAM: So I'm just going to draw the two examples that you gave us. So if it really looks like a U wave, it's distinct, we're going to take QT just like this. But if it's a weird looking T wave, possibly the U wave is in there, it's distinctly bifed, then we're taking the end of this. Is that fair, Mike?
MICHAEL ACKERMAN: Exactly. And that one, Dr. Asirvatham showed very nicely, because there, if that were a U wave, that's a little bit too much fusion of the U wave into the proximal proceeding T wave with that amplitude point there, the so-called T1 and T2 being greater than half of the amplitude of the T1 proximal T wave.
And as that amplitude of the second hump gets lower and lower, then that becomes more U wave-ish. Where it's the easiest is actually when that T2 amplitude is of greater amplitude than the T1, like that, and that is never a normal U wave. And that is a highly suspicious LQT2, a specific genetic basis, for that T wave morphology.
So here, he has normal T waves all throughout. And I think this is what we get at, John, and I think what you suspected yourself was, could we be falling prey to a false positive Sammy stretch? So this was popularized by a good friend of mine, Dr. Sammy Visconti, with the stand-up test, where upon standing, then the heart rate has a brisk chronotropic response, and the QT didn't adapt in a heart rate appropriate way, leading to the Sammy stretch, a lengthening of the QTc.
Now it turns out that that can sometimes detect concealed long QT syndrome, especially LQT1. But when that QT standing reaction isn't supported by a maladaptive QT reaction in the recovery phase, then that Sammy stretch in the first minutes of standing could well be a false positive. And we and others have shown that it can be a false positive in young people up to 20% to 30% of the time, and that false positive rate is even higher if there's not complementary altern-- maladaptive pattern at the 3 minute recovery phase, because what this is really getting at is catching LQT subtype 1.
And so if it's a Sammy stretch in the first standing position and at peak, the QT is still wrong, which is sometimes hard to measure. But then in the recovery phase, the QTc is still above 470, at 2, 3, 4 minutes of recovery, then it all fits, and you say I've got a gotcha moment, I've caught LQT1. Which in this case, you might have caught it, but then I would say that probably isn't the blame for the faint in the first place, because if that was an LQT1 triggered faint, his resting QTc should never be that good at 414 milliseconds.
But the exercise test is a standard test in the evaluation of genetic heart rhythm diseases. It's a critical test, especially for genetic test result confirmation, and so-called VUS resolution, variant of uncertain significance, which we'll come to, especially for the other condition, which his story could fit with, which would be CPBT. Which if this were channelopathic, you would have expected with that normal of an ECG at the beginning, this would be way more likely to be CPBT than catching concealed LQT1 with the Sammy stretch, if that makes sense.
SAMUEL J. ASIRVATHAM: Thanks. Great. What else did you do, John? Thanks, Mike.
JOHN ROSHAN JACOBS: Thank you. So this gentleman, he came about a week or two later to us, and he was very disturbed by the fact that he had noticed that he felt palpitations on exertion. And this was particularly when he went to the gym or when he went hiking. And he said it was not a persistent increase in heart rate, but he felt a few extra beats, which sort of disturbed him. And on a couple of occasions, he rushed to our emergency when he had these symptoms, but his ECG came back as normal.
He then got himself a Apple Watch which was monitoring him, and on that, he noticed that--
SAMUEL J. ASIRVATHAM: So John, if I understand right, the palpitations that he's talking about are not like regular tachypalpitation?
JOHN ROSHAN JACOBS: They were not.
SAMUEL J. ASIRVATHAM: [INAUDIBLE] sytopic beats.
JOHN ROSHAN JACOBS: Exactly. Exactly. But it left him very disturbed because he said it felt like a fluttering inside, some extra beats. He was very uncomfortable with it. And he, being a doctor himself, had done a lot of reading on the fact that he had an exercise-related syncope. So he was maybe--
SAMUEL J. ASIRVATHAM: I'll just make a comment here, John. When you think about tachypalpitation, exercise, young male, we think outflow tract VT. So question that comes up sometimes is can outflow tract VT or outflow tract PVCs cause syncope? And just a couple of things there, is usually outflow tract VT does not cause syncope. People get it, and they feel OK.
But just two variants to keep in mind. Sometimes people from very distal outflow tract VT, like from the suprapulmonary region, can get polymorphic VT because of varying exits from that side. And second is in the athlete who has low resting sinus rates, sometimes they go into an outflow tract VT, they start biking or they stop exercising, the VT breaks, and that sudden drop in heart rate can make them feel dizzy or possibly faint.
PVCs themselves, can they precipitate? The answer is yes. One of the mechanisms for the neurocardiogenic syndrome is cardiac stretch receptors, mechanoreceptors, which just pound for pound are more important in the right ventricle. And this is probably the reason why patients with acute PE, also patients who have pulmonary hypertension, sometimes get neurocardiogenic syndrome. You can get a PVC, you get this post extra systolic potential, potentiation, and that can precipitate a neurocardiogenic syndrome.
So you don't kind of say unrelated, but may give you another mechanism, something to think about and [INAUDIBLE] effect. OK, and then what happens?
JOHN ROSHAN JACOBS: So we knew that he actually had something, in terms of when he had his episodes. He did actually have some PVCs as we saw in the trace, so we got a Holter done, but the Holter just showed 0.1% monomorphic PVCs coming from the outflow tract. And at this juncture, we also looked at his echocardiogram, and that was because he had an echo done elsewhere, in the Center of [INAUDIBLE], where he was told, where they initially felt it was slightly abnormal, but later on, the experts got together and ruled it out as being a normal variant.
So he also had a genetic testing done elsewhere, the reports of which came in showing a KCND3 variant, which was suggestive of Brugada. Now we felt this was probably just a red herring over there. We did an ECG with the V1 and V2 being recorded at an interspaced above, and the ECG was absolutely normal.
SAMUEL J. ASIRVATHAM: So maybe this will be an excellent point to get Mike to comment. So as I'm sure you get a lot of questions like this clinically and from all over the world, someone's got the genetic testing done, and they get a report like this. Just your approach in general, and things that non-expert electrophysiologists should keep in mind in approaching a case like this.
MICHAEL ACKERMAN: Yeah, thanks, Sam. I think this scenario is why I have way less head of hair than Dr. Roshan, because it just drives you crazy. Because we try to say if you don't know the phenotype, don't go fishing for the genotype. So you get in trouble when you say, I'm not even sure what the patient has, I'm not sure what I'm going to call him or her, but I'm just going to go do the whole clinical exome anyway and see what comes out.
And then, if it falls in the wrong hands, you could see, Sam, how quickly this could be then say, hey, I've got it solved. He's got a likely pathogenic variant in the KCND3 Kv 4.3 potassium channel, the Ito current. It's been previously published, it's been functionally characterized. This seems weird to me, but this gentleman just had a Brugada syndrome episode during cycling.
And then forget how everything started, right? And forget that my whole phenotypic index of suspicion was lacking. My tests weren't confirmatory, but I guess the genetics is the truth, and therefore try to tie it together, in which, John, you were so nicely showed, how you were skeptical of this.
And I think that's one of the important lessons for clinicians. When we read a genetic test report, or interpret a genetic test report, that's not-- interpreting a genetic test result isn't called literacy. Can I read the report? It's called-- just like decoding the ECG isn't called reading the report at the cross of the top of it and seeing what it said the patient's rhythm was and so forth. We look at it. We interpret the data.
Now many of us, we just don't have-- that's not our language. The language of genetics is foreign to us. And so we then acquiesce to the genetic test company and just read like we read here and say, oh, it's a mutation, it's Brugada syndrome gene, it's called likely pathogenic, therefore it must be, instead of looking under the hood and doing the due diligence.
And it's just fortunate for me, John, that I just so happen to know this mutation because my team discovered it 10 years ago. My PhD student, John Giudicessi, who's going to be one of Sam and Christopher and my new genetic cardiology partners at Mayo Clinic come this summer, when he was a graduate student, we found this. We found this in a case of Brugada syndrome. We found it in a case of idiopathic ventricular fibrillation. We functionally characterize leucine 450 phenylalanine.
And in the dish, it's fascinating because it causes a profound increase in the Ito current. And whether you have loss of INA in phase zero, or gain of Ito in phase one of the action potential, you can get that depolarization, initial repolarization, mismatched, in other words, Brugada syndrome substrate.
So it all fit. It all made sense, until 10 years later, what do we now know about this variant? Well, we know that this variant, in the public genome aggregation database, is present in looking at over 140,000 humans, this specific variant is present in about one out of 4,000 of us.
And the beauty of genetics is one thing that beats genetics is mathematics. So the math doesn't lie. There's no way this variant at a 1 in 4,000 frequency can be a monogenetic substrate for anything, right? Brugada syndrome is 1 in 5,000 people. KCND3 mediated Brugada syndrome is at most 1% of all Brugada, so that's 1 in 500,000.
So this single variant in this gene, even if the gene is a Brugada gene, or an early repoll gene, is 100 times more common than the collective frequency of all KCND3 mediated sudden cardiac arrest syndrome disease combined. So it can't be.
Now, could it be a functional risk allele, a modifier, a pro-arrhythmic polymorphism? Maybe, but it can't be the cause. And so, I'm so impressed, John, that you were very skeptical and say, this doesn't fit, but since it's in the Brugada pathway, let's take a closer look and see if we incidentally have stumbled upon Brugada. And then you probably did a high lead ECG to see if you could bring out a Brugada pattern and so forth.
But I think the most important thing is what you did, is you said, I'm not sure I'm buying this result. It, at most, is an incidental finding, because it surely doesn't connect with what happened to this patient while cycling.
So great job. I wish I could say that that's the norm out there. It unfortunately isn't. I've seen way too many patients with this exact scenario leave with the diagnosis of Brugada syndrome, exertional syncope Brugada syndrome, and a defibrillator, when this genetic marker had nothing to do with anything.
SAMUEL J. ASIRVATHAM: Thank you, Doctor. What other findings did you have here on this patient, John?
JOHN ROSHAN JACOBS: So we heard from him that the doctors who did his echo elsewhere were not sure whether it was completely normal. So we got his echo done, and the outflow tract did have some kind of bulge in it with some dyssynchonous contraction, which our echo expert felt was not in keeping with normal. All the same, he felt this was something that could easily be missed, unless somebody really looked for it.
SAMUEL J. ASIRVATHAM: So, John, was an MRI done on this patient as well, or was this maybe an echo finding?
JOHN ROSHAN JACOBS: Yes, yes. No, so this was an echo finding, and we did an MRI as well after this. So the MRI images showed some amount of cremations in the free wall of the RVOT. And our radiologists also admitted that it was a subtle finding which could have been overlooked, and they weren't very sure whether this would be deemed as a normal variant. But they felt that they don't usually see these kind of cremations.
SAMUEL J. ASIRVATHAM: So maybe, John, I can just share something here. This is also a very common type of question that comes up from the ablation world, is while doing an outflow tract ablation or restratisfying a patient, it's usually this portion right here that will be described as a pouch. And sometimes, it can actually be one, but it's the unsupported area of the right ventricular outflow tract.
Notice, like as you go more to the science, you have some supported structures. And this is a little thinner than normal. And it is a place where our catheter falls into, we can perforate, and can actually look like a pouch. Posteriorly, also, there is an unsupported area, this transverse sinus region, but that region for the outflow tract is very far. It's very distal.
So what happens is posteriorly also, we have the outflow tract, is a free structure. But on the back, we've got the aorta that's just bulging into here, so that will almost look like a reverse pouch or a bulge in that region. But here, that leaves it, everything else looks like a cylinder, but here it looks like it's a pouch that comes in there. It is a place that has a lot of trabeculation anteriorly, and it's so different from posterior, where it's smooth, that it could get described as like a cremation or an area that has a lot of trabeculation.
So it is a normal structure, it's a normal finding in this region. Some of the clues, when this really is a diverticulum or a pouch is the absence of cremation. So if we don't see the trabeculation in that area, and you see a discrete outpouching, it's probably real. It's often will be squished right underneath the sternum, something that doesn't happen with the normal pouching here. And if those things are seen, that may be something we really have to evaluate, and see could it be a substrate for PVC or VT in that patient.
Please go back to sharing your slides with us also and see. One of the questions that came up, both for you and Dr. Ackerman, is like in this patient and in others, what's the role for noninvasive testing for restratification? I think things like T waive alternates testing, signal average ECG. I don't know if this patient had any of that or not, but even if otherwise, just as a general comment.
JOHN ROSHAN JACOBS: We did signal average ECG on this patient, and it came back as normal.
SAMUEL J. ASIRVATHAM: Do you have an example of the signal average ECG?
JOHN ROSHAN JACOBS: I could just put it up in a second.
SAMUEL J. ASIRVATHAM: OK. And meanwhile, maybe I'll ask Dr. Ackerman to comment. Mike, just in testing role for T wave alternates testing, signal average ECG, I know internationally practice varies quite a bit for this.
MICHAEL ACKERMAN: It does, and it's quite debatable. Even for the signature lesion where we use signal average ECG the most historically in genetic heart rhythm centers, namely ARBC, the signal average ECG is falling a bit out of favor. In fact, the Johns Hopkins ARBC program, they seldom utilize it anymore. Again, too many false positives, uninformative.
And I think this is a good example of if there's a really bothersome story that you, Sam, and all of us are just, we just cannot let ourselves call it a vanilla faint, then I'd order every test I can think of, kind of like pit bull tenacity. But when the story is weak and hedging towards a nonarrhythmic mechanism of fainting. And then we start going to esoteric tests, if you will.
We just so quickly can get into false positives, and I think we have already now three examples, John, of what I call FUS's. And we're making a lot of fuss out of FUS, findings of uncertain significance. So a lot of cardiologists are really harsh on the VUS's, the variants of uncertain significance, and say, oh, genetic testing is terrible, I got another VUS.
But we wrestle with FUS's all the time. Was that Sammy stretch in standing? Was that a pathologic signal? Was that a false positive noise? The echocardiac imaging is that a pathologic abnormal RV outflow tract pouch, or is that just noise? That's an FUS.
And here, there's very few things-- I guess if the signal average ECG was convincingly positive, then that would keep the possibility of ACM or ARBC-triggered fainting in the setting of him cycling as a possibility. But it's of almost no use, well, very little use, outside of the possibility of arrhythmogenic cardiomyopathy.
T wave alternans, if it's macroscopic and obvious, it's kind of easy. And to get clearly in long QT syndrome is an important risk harbinger, but it's one where you almost never see macroscopic T wave alternans, like almost never.
SAMUEL J. ASIRVATHAM: So maybe I'll ask Dr. [INAUDIBLE], one of our superstar Fellows, just signal average ECG, we get it in our practice, some people more than others. And you know, Dr. Ackerman has told how it can be of limited value. Do you want to interpret this for us, and then what situations you think it might be of value, Roshan?
JOHN ROSHAN JACOBS: Sure. So for the signal average ECG, we are trying to basically look at how the terminal portion kind of activates in a QRS, basically averaging a number of cases.
SAMUEL J. ASIRVATHAM: Looks at the terminal portion. In other words, it's like how we, in a substrate map, when we're looking at PVCs, VT ablation, and restratifying, we want to see what are those late signals, so those late potentials. And we have a way to try to pick this up. Maybe I'll just briefly describe this, a very commonly asked question.
So when we do substrate ablation, one of the big things we're trying to figure out is sites of late activation. So diseased myocardium, but viable tissue. So viable tissue, disease myocardium, re-entrant to VT. And if we're trying to predict what that is, we have to see is there this late activating signals.
What do we mean by late? Is it's occurring after the rest of the heart has depolarized. So it's after the QRS, or towards the end of the QRS, separated from the QRS. When we have electrodes in the heart, we can find these pretty easily. But on a surface ECG, how do we find it?
So in a surface ECG, if we're to find them, they're so small, micro voltage range, you have to gain it up. And if you gain that up, your ambient noise is going to gain up. So it becomes impossible to really interpret and say that these signals are present.
And that's where this concept of the signal averaging of the ECG comes. So signal averaging is like you're essentially filtering out noise. You start with a filter, like a high pass of 40 Hertz or something like that, and then you do a template match for each QRS. So you just find some fiducial point to tell you that's what we're going to take, beat after beat after beat after beat, and signal average, or take the arithmetic sum of all of these beats.
And how many beats do you need? By doing this arithmetic sum, all the noise will cancel each other out because you'll have as many positives and negatives. And until you get a zero noise level, you take beats. And usually, if you have 40 Hertz as your high pass, that's about 200 beats that you need, or about seven minutes of signal averaging.
And then what's left behind, if it's late and it's significant, you say it's a positive study. And three things that you look at is just the filter ECG itself. Is it more than 114 milliseconds? 110, 114. That's telling you there's some delayed activation somewhere.
Second, you'll take the root mean square of your terminal 40 milliseconds of the QRS. This root mean square is you're just kind of averaging out those voltages. And if that end of the QRS is less than 20 microvolts, that means the later part is diseased. So late plus diseased, you're thinking a substrate.
And then you also will look and see the terminal time, the last part of the QRS, the last voltages that you see, the last small voltages. How long is their duration? This is the most important aspect when we look at VT ablation, because this is telling us it's not just outliers, but there's very slow conduction to these outliers.
So sometimes, you'll get normal in two of the variables. The unfiltered QRS looks normal. The root mean square looks normal. But if you're late activation is for a long duration, that's something we use. So Dr. Ackerman pointed out, like limited utility for when for restratification in the inherited world, but gaining popularity again in the ablation world as a marker for, have we done complete substrate ablation?
So you look at these late activating, you keep ablating, and then have we lost all those outliers? So a lot of things. One important thing to kind of also keep in mind here is why it fails, and why do we get this and it's not really a marker, why can't we find it? Maybe I'll share a slide here, if I'll get it.
So just to kind of explain how this can be difficult with just this time domain averaging, temporal averaging. So here, abnormal signal before a PVC, here if you look in the sinus rhythm, it's just part of this electrogram, part of the QRS. This is one of the difficulties with signal averaging. We may have abnormal signals, but it's just not late because you have Purkinje network that can get you to different sites at the same time. So this is one of the reasons some groups have looked at, like Sanjeev Narayan and others have looked at like spectral analysis of the signal, to try and find the abnormal signals, even within the QRS.
Another issue is we can see hugely abnormal signals very, very late. But they're so late that they're not going to come in the times we normally look at signal averaging. They may even be so late that it becomes part of the P wave that's being measured over there.
Another important issue is bundle branch block. The value of signal average ECG goes way down when there's bundle branch block. And the reason is clearly late signals in this portion of the heart, but if the QRS itself is long, it's just going to be hidden within the QRS. And that's why another type of signal averaging that's also gaining popularity is spatial averaging, not looking at the time, but having multiple ECG vectors, like with the body vest, multiple ECG vectors, and then averaging so that you just see what are the later abnormal signals. It doesn't matter how wide the QRS, or where the QRS is.
So just some things to kind of keep in mind when you're asked to interpret a signal average ECG. Do you have any follow up or further testing on this patient?
JOHN ROSHAN JACOBS: Yeah, yeah. So that's one other thing to mention, was this gentleman, I noticed, was slightly thickly built. He admitted to having nocturia, and not feeling very fresh during the day. So I did get a sleep study done for him, and it actually showed significant sleep apnea with an AHI of 14.8, and this improved with CPAP. So he was started on CPAP therapy.
However, he continued to complain of these fluttering-like beats that happens when he exerts himself, like goes to the gym or climbs uphill or cycles with vigor. And the patient was keen that he should be further evaluated for the same. And he was also very anxious about the fact that there was this suggestion of a long QT elsewhere.
So actually, it was more on his prompting that we went ahead and did an epinephrine challenge for him. And when that was done, interestingly it induced a lot of PVCs, which are monomorphic, similar to what was seen on the Holter. So in somebody who had come multiple times to the emergency with fluttering, the ECGs were all normal at that time. On the Holter, we picked up a total of 120 PCVs over 24 hours. On epinephrine challenge, we had every fifth beat being a premature ventricular contraction. And he perceived every single one of them.
SAMUEL J. ASIRVATHAM: Maybe, John, before you continue, I'll just ask Mike for an opinion, and just general rationale of epinephrine testing, epinephrine challenge. And then maybe, Siva, if you could comment on thoughts on epinephrine provoking PVCSs mean anything to you. Do you approach any differently? Do you use this agent in the EP lab to bring out PVCs? Maybe Mike first?
MICHAEL ACKERMAN: Yeah, so the epinephrine QT stress test was really developed at the same time, now almost 20 years ago, by us at Mayo Clinic, and by [INAUDIBLE] Shimizu in Japan. And so there were two different epinephrine stress test protocols. Theirs was the bolus protocol. Ours, the Mayo Clinic epinephrine QT stress test, was starting at a very low infusion, 0.25 going to 0.5, or 0.05, 0.1.
And really, at its best, it was a way to confirm LQT1 pathology, because you would get a paradoxical reaction, or a paradoxical lengthening of the absolute QT interval. It was a beautiful way to catch long QT syndrome subtype 1, particularly back then in 2004, when you would send a blood sample off, and you'd get a result back in 3, 4, or 5 months.
And now it's almost an obsolete test, because we've learned that you can get almost exactly the same information plus more out of the stress test. And so when we used to do a lot of epinephrine QT stress tests from 2004 to 2015-- we do very few now in the last six years-- it may have a role in sudden cardiac arrest survivors, where they're not ready or are unable to get on a treadmill to do a stress test. It may have a role to be sort of a catecholamine provocation for CPVT as well, although isoproterenol may be a better challenge test for CPVT.
So I would say in the person able to do a stress test in 2021, there's almost no reason to do an epinephrine provocation test any longer, even though we pioneered it here at Mayo. It had a great role 15 years ago. That role is now replaced, almost don't need it, only use it for unexplained sudden cardiac arrest survivor evaluations, I would say, at the present time.
And part of the reason it was so many people were misinterpreting the maladaptive paradoxical QT reaction because they forgot to realize that if somebody has a normal U wave, or even more of an impressive U wave, under epinephrine that U wave will get so accentuated and fused and incorporated into the proximal T wave, that it will look like a pathologic T wave. And if you didn't recognize the U wave at rest, and then thought it was a bad T wave with epinephrine, you're going to say, oh my goodness, that was dramatic QT prolongation, when all it was was epinephrine mediated accentuation of the normal U wave, especially in the septal profile leads of, say, B3 for example.
So we loved it when we invented it. It's sort of now almost extinct.
SAMUEL J. ASIRVATHAM: Yeah. And so Mike, just to also add to that, there's also this [INAUDIBLE] observation from some time ago about epinephrine, and you get these funny looking TU fusion as a marker of [INAUDIBLE]. But just exactly as you said, if what's being noticed is the T wave is fine, but the U wave just gets bigger and bigger and approaches the T wave, that's a perfectly physiological response to epinephrine.
On the other hand, if we see the T wave is getting longer and longer and longer, the QT, and then that produces changes in the morphology of what was already a long QT, then that this could be a precursor or [INAUDIBLE] type event.
MICHAEL ACKERMAN: Absolutely.
SAMUEL J. ASIRVATHAM: Siva, just your thoughts on this patient, epinephrine giving lots of PVCs, mean anything to you? Do you use it in the EP lab?
SIVA K. MULPURU: Epinephrine in the EP lab is not the first line in our practice. We try with isopropyl first, and if you're able to bring out the PVCs, we already have the answer. One of the issues is if you have an increase, if you want to increase parasympathetic tone, some PVCs are more predominant when you have a heightened parasympathetic tone. You can use phenylephrine to bring PVCs in those patients.
Lastly, if those two we cannot bring with PCVs isopropyl and phenylephrine, you could try epinephrine, but it is not my go-to agent.
SAMUEL J. ASIRVATHAM: Yes. So sometimes, people like epinephrine, when isopropyl, you have a specific problem that is it's the sinus node sometimes, it's just more sensitive. So if you see the PVCs we're trying to get, but sinus rate speeds up so much that it's fusing with the PVCs and it's hard to map. Sometimes, folks will try phenylephrine along with isopropanol just to get some sinus node suppression from a reflex vagal, or epinephrine instead of thing.
But is it fair to say you think, Siva, that this suggests that outflow tract VT might be an issue here, or caused triggered PVCs outflow tract and epinephrine bringing it on? I think, without doubt in this patient, you'd probably put an event monitor of some sort, maybe an implantable loop recorder, to see when they get the event, what it is.
But in addition to that, I guess the question that would come up, and I'm sure it was a question for you, John, is should you do a restratifying EP study? So maybe I'll ask Dr. Ackerman for an opinion, and then maybe Ammar as well to see should we think about restratifying, or is it OK to do an event monitor? And maybe, an additional question, any role for tilt table testing? Mike, your thoughts?
MICHAEL ACKERMAN: Yeah, I think this would be a time to do a big regroup and say what do I even think is going on with the patient in my concern? And I would say so far, we have basically ruled out long QT without the genetic test. We can say that was a false positive U wave reactivity. We've ruled out CPVT from a practical standpoint, with both the stress test and the epinephrine test.
We've ruled out arrhythmogenic-- so we've already ruled out the big bad things. And even felt from the beginning that it probably wasn't arrhythmic fainting anyway, with some of those key findings that John shared with us, and that this could be neurocardiogenic, vasovagal, albeit during exertion, which is far more common than what people realize.
So I'm not sure I would've keep pressing on with further testing per se. I definitely would be doing a loop recorder. I would have done the loop recorder in my own practice after the first exertional faint, because I might have, if I would have caught him earlier, I would have said, you know, this isn't feeling ominous, we've done our due diligence. But if you were to do it again, rather than just my mental assessment, I'm going to have an independent rhythm spell arbitrator with the loop recorder.
So I love it for that. I think we wait way too long to utilize implantable loop recorders in some of these inadequately explained faints. So I would have voted for that. The tilt table test, no, because I don't really care what the result would have been. If it was positive, yes it fits with my thought. If it was negative, it still doesn't discourage my thought. So I think the utility of the tilt table test in a young person is getting less and less.
I'm OK if we thought maybe we should come to the lab because we might be dealing with stumbled upon outflow tract PVCs, even though I wouldn't be totally convinced yet that had anything to do with his actual faint mechanism, but potentially would be heading towards getting rid of them and getting them out of the equation anyway.
That would be some of my thoughts. I'd love to know what, Siva, you or Christopher or others have.
SAMUEL J. ASIRVATHAM: Ammar, your thought for EP study? So issues here are we comfortable with monitoring, or there's been this syncope, there are these PVCs, and there's this possible pouch in the outflow tract?
AMMAR K. KILLU: Yeah, I think it's a very good question. It comes up a lot, as Dr. Ackerman said. Definitely needs discussion between the various stakeholders. I think we have to be careful not to take someone to the lab and be overly aggressive with stimulation, and then induce something that's non-specific. And then we're left with something that we could have gotten in pretty much anyone, and then end up treating that and saying that's the answer.
So in this situation, I think ambulatory heart rhythm monitoring is very reasonable. I agree, though, with the PVCs. It makes sense that if they're bothersome to the patient, then targeting those with ablation, and then performing controlled EP study would be reasonable. And by that, I mean avoiding overly aggressive, overly tight triples, or something like that, where you may get a false positive VF induction or something like that.
SIVA K. MULPURU: One of the things that I have learned from our exercise physiologist, Dr. Allison, in patients who have these multiple exercise syncopal spells, especially their endurance athletes, he makes them bring their bike into the lab and simulate that exercise that the patient is doing. So this patient is doing 35 miles, he makes them do it. And a lot of times, you may be able to bring out the patient's spell by reproducing the exercise conditions. The [INAUDIBLE] treadmill may not reproduce what is undergoing during that extreme conditions.
SAMUEL J. ASIRVATHAM: So that would be better, Siva, than an event monitor, with them exercising, because maybe less noise and we can change the leads and things like that. So yeah, and just to emphasize what Ammar said, your overaggressive EP study is two aspects. One is just doing four or five extra stimuli, multiple drugs, but it's usually overinterpretation. Just about anybody you can get into VF, but we don't get anybody into VT. That's one.
Second is, you will not get with extra stimulation testing a induced PVC that then gives you a polymorphic VT. Those are both abnormal responses. So I think the one that would bother me in this patient, as this patient were to die suddenly, this could be an outflow tract VT variant, where it's so high in the outflow tract, the origin, that we can get polymorphic VT because of this multiple exits.
So if that's still playing in our mind, we're worried about it, I think that could be a potential rationale. But we would really pay attention to what ectopy am I getting? Where am I stimulating from? And I don't want to see just a non-specific response. I think it would be reassuring in this circumstance if we don't induce anything. Be a choice discussion with the patient, but that would be the rationale.
But maybe, John, you can just give us a quick update, and then we can wrap up for today.
JOHN ROSHAN JACOBS: Yeah, so we had a discussion with a patient. He was keen to go ahead with an EP study. And we did an EP study. We were not able to induce any arrhythmia whatsoever. The other thing that we interestingly noticed was that just like how none of the treadmills brought over any PVCs, but epinephrine brought PVCs. Even on the EP study, isoproline did not bring out any PVCs. But a slight dose of epinephrine did definitely bring out the PVCs.
The other doubt that came to my mind was the patient was not keen on having a loop recorder, and he had a smartwatch, which he continuously monitored. So I was wondering if that could suffice, since he anyway wasn't keen on having a loop recorder.
SAMUEL J. ASIRVATHAM: So maybe, Mike, now you know that's almost an immediate question from a patient, when we say loop recorder. Your thoughts on equivalence in our Life Core, Apple Watch, something with an ECG trace that is not just pulse rate?
MICHAEL ACKERMAN: Right. I think in this case, John, I would have simply asked this gentleman and say, the two times that you've done your faint so far, would your Apple Watch have caught those? And the answer is no. And so I would have helped him to become keen on the implantable loop recorder because I don't think any of our smartphone-enabled mechanisms would have caught him doing what he was doing in the setting in which he was doing it.
So I don't think it's a reasonable alternative. I would have tried to help him understand that, yeah, I get why you don't want a little skin nick for the loop recorder. But if we're really going to catch a third spell, if you're going to do it again, your smartwatch would not have caught it.
JOHN ROSHAN JACOBS: The other thing is should I ask him to refrain from exercise? Because he loves going to the gym, he loves biking 35 kilometers, which I have told him to hold off. We've started him on a beta blocker, and on beta blocker, he says he feels a lot better.
Though he did say very recently, about a month into being on metoprolol 25 milligrams twice daily, he told me that when he went climbing uphill, he felt the same fluttering happening. But that apart, he'd been mostly asymptomatic the entire last month. We did offer him an option of canceling an ablation with epinephrine to bring out the PVCs if they were so bothersome to him. So what would be your suggestions in terms of considering the beta blocker, and whether we would allow him to do exercise, or should we caution him with regards to the exercise that he does?
MICHAEL ACKERMAN: Yeah, I would never restrict exercise for a non-arrhythmic condition or a nonlethal substrate, so I'm under-- I'm not concern that you've missed a potentially sudden cardiac death predisposing channelopathy or cardiomyopathy. I think this is tilted way towards normal, nuisance, annoying neurocardiogenic syncope during exertion.
I would keep him active. I think I would try to get the loop recorder implanted. I think that's the best intervention that he needs. I like beta blocker a lot for some of these outflow tract PVCs. And Sam knows this better than me, some of our patients, a whiff of natalol, for example, I mean a full-size adult on 10 or 20 of natalol, just causes this palpitations to vanish. And so, some of these kind of PVCs are ultra-beta blocker sensitive. No downside of that.
I would also have no problem as a non-invasive genetic arrhythmologist asking my interventionalists to get rid of that offending PVC and get this out of the equation. But shutting him down and having him become sedentary for what probably is a nondangerous substrate, I would definitely not advocate.
SAMUEL J. ASIRVATHAM: Thanks. Thanks. Thanks a lot, Mike. Well, it's been a really nice session. Mike, thank you very much for joining us. It's just an absolute treat to have you with the insights that you share with these cases like this. It's something that us in the EP community just get way too little of and really appreciate it.
John, all of us know what you're going through there, and thank you very much for taking the time and presenting the case as well.
SIVA K. MULPURU: Thank you, Dr. Ackerman, for joining us today for this excellent live session. I think we had a great discussion. We answered all the questions. And there's one question that was submitted by a physician who had a patient who had a ventricular fibrillation arrest. Patient had a structurally normal heart. Apparently had some PVCs on the rhythm strips upon arrival at the hospital. MRI was completely normal, and on genetic testing, he had a pathogenic mutation detected on NYH7 gene.
He subsequently underwent an ICD implantation. He had recurrent ventricular fibrillation that was thought to be PVC initiated ventricular fibrillation. He underwent a moderator and PVC ablation, and subsequently was started on creatine.
So he had two sons, the first degree relatives who had the same mutation. And how would we approach this? And what do we counsel this particular patient and his family?
MICHAEL ACKERMAN: Yes, Siva, we did have a great session together. I was so glad to be with you all. And this one, actually, we could probably spend a long time on, unfortunately, because it's actually a little bit complicated and convoluted in the sense of-- it ties in with John's story, doesn't it? The story. What is the index of suspicion? Are we going to call this patient NYH7 mediated disease, which I think are the question, or probably meant the gene MYH7, which is the gene that encodes the beta myosin heavy chain, which is one of the two most common genetic subtypes for hypertrophic cardiomyopathy, or HCM.
And so if this is MYH7 mediated HCM, then his question is really good, which is can I have MYHCM mediated VF before I show overt signs of HCM, or maladaptive cardiac hypertrophy? And that answer used to be no way, as in never. You would always do hypertrophy if you have MYH7 mediated disease before you would do arrhythmia.
It's changing a little bit. And where we're learning that change is not in HCM, but in arrhythmogenic cardiomyopathy, where we and others have published that you can do VF before you do overt signs of a structural myopathy, and in particularly with PKP2 mediated ACM. In other words, you can have a precardiomyopathy electropathic expression of the disease.
We haven't seen that as much with HCM. Probably some of the exceptions are with some observations of troponin T mediated HCM, where you can do pretty significant ventricular arrhythmias with negligible hypertrophy but a lot of disarray, but not no hypertrophy. So this again would make me pause and say, is that really a pathogenic variant? Or is it potentially background noise?
The observation of apical cardiomyopathy, or apical hypertrophy, in one of the variant positive sons could fit, or you and I have seen plenty of over diagnoses of atypical hypertrophy. So it depends on which came first. Did the genetic result come first, which then the ultra sonographer cardiologist knew about and looked into the echo a little bit differently than what they might have if they didn't know, if they weren't led with that information?
So I'm not quite pleased that the story is hanging together in this really important question, because PVC-triggered VF that you would choose to go after the target and ablate is something that we seldom see in genetically mediated hypertrophic cardiomyopathy. So I think we would need more details.
If I was convinced that it was a true disease causative variant, what would I do in the variant positive sons? I would do full cardiac evaluation, assessing the degree of phenotypic expressivity by our electrical test, ECG Holter stress test, by imaging echo and cardiac MRI. And if the phenotype was negligible, I would observe. If the phenotype was impressive or suggestive, depending on how old those sons are, then the father's sudden cardiac arrest episode would be a personal risk factor for the sons.
But I would be a little bit slow before I commit this family to a diagnosis of beta myosin heavy chain mediated HCM, where the VF preceded the H, the hypertrophy. I think we have some loose ends that we need to tie up first.
SAMUEL J. ASIRVATHAM: Thanks. Maybe it's also a quick good opportunity just to-- it's a tough population, like not this patient, but hypertrophic cardiomyopathy patients for the invasive electrophysiologist. So story would be clear cut VF, plus or minus PVC trigger, has an ICD, has frequent shocks. And then the question becomes, how are we going to minimize shocks in this population?
Maybe we'll do a quick round of everyone here for comments. And then, we can wrap up today. Maybe start with Chris. Chris, thoughts, your approach here. Very tough, unfortunately. We definitely get the patients, and we try our best to help them. But your approach here?
CHRISTOPHER V. DESIMONE: Yes, definitely. And I think what muddies the water is the genetic test is done and it's there, and now you can't ignore it.
SAMUEL J. ASIRVATHAM: Here, I'm talking about clear cut hypertrophic cardiomyopathy, [INAUDIBLE], and then to approach thoughts as an invasive EP.
CHRISTOPHER V. DESIMONE: So definitely beta blockers would be my first go-to. Depending on the age and depending on how much I need to stop the shocks would be either ablation and/or amiodarone until I let things settle down. That would be my first go-tos. So especially if I'm thinking this is PVC mediated.
If I think it's substrate mediated, or if this patient has this apical or pouch or things like that, again, I would go to ablation, and then amiodarone probably in the post-operative period until I know it's settled down, because I don't want him to keep having recurrent shocks.
SAMUEL J. ASIRVATHAM: OK. What about, maybe, Siva. Your thoughts?
SIVA K. MULPURU: So I think if it is PVC-induced ventricular fibrillation, it's important to understand the trigger for the ventricular fibrillation. Either 12 lead ECG tracings, when he's having lots of these PVCs, or using the defibrillator to understand where the location of the trigger is. In this case, looks like the trigger is from the moderator band, maybe some Purkinje abnormal signal there was modified. So it's important to follow this patient long term. And for many years, if it doesn't have any shock, it's probably just a run-of-the-mill PVC, Purkinji-induced ventricular fibrillation, rather than this gene-related arrhythmias.
SAMUEL J. ASIRVATHAM: [INAUDIBLE], and maybe I'll just point out a couple of things, is we talk about hypertrophic cardiomyopathy substrate. It's just like how we restratisfy with an MRI on these patients. There's several mechanisms for ischemia and hypertrophic cardiomyopathy. There's often subendocardial ischemia from wall stress, and these patients can have subendocardial, myocardial slowing. But the Purkinje network is spared because it can be very resistant to ischemia.
So a given PVC trigger from the conduction system has got like a pretty worst case scenario, when you have neighboring areas that are able to complete these circuits. You can actually get areas of ischemia, severe ischemia, just from myocardial stress strain, demand-supply type relations. And you can also have some patients who have intermyocardial course of hypercardial coronary vessels, so bridging in this patient population.
Just more, I'm more eager to think that ablation will help. If we have one of those in MRI that shows this patchy scar, shows an atypical aneurysm, if you also see evidence that there's an area that appears kind of desynchronous, and a pattern, like Siva said, of each episode starting with a VT and degenerating, then we get like a little bit higher-yield type scenario.
Clue to myocardial bridge in patients with hypertrophy is if they have stress-related VF. So it isn't so much the PVC trigger, but you put them on a treadmill, and they get VCF, and it's almost at a certain fixed level that they're getting it, be suspicious, look carefully for bridges, and occasional patients that can be surgically kind of corrected.
Maybe one last question for you, Mike, in addition to being an expert in this area of inherited myopathies and triggered arrhythmias, you're also a professor of pharmacology, and your depth of knowledge of drugs is really of great value. Just in for hypertrophic patients, managing arrhythmias, any drugs that you just don't like using, or drugs that you favor, maybe a little bit safer in this population?
MICHAEL ACKERMAN: Yeah, it's a great question. I think the pharmacology of HCM mediated arrhythmias is disappointing overall. I know, I think, Chris, you already mentioned the ones that I agree with for beta blockers and amiodarone. I think overall, amiodarone is not that therapeutically efficacious in these patients.
I think the one setting is not pharmacological, but really important to mention is, I've had several young hypertrophes now, where their VF episodes are clearly catecholamine triggered. And so they're not like the scar you're talking about, or the potential bridge, where we have done what is an incredibly therapeutically efficacious intervention in long QT syndrome and CPVT, what I'm getting at is left cardiac sympathetic denervation. And we've done that now in more than a handful of hypertrophes with very satisfying results.
So again, it's sort of where you get the impression that every one of their episodes is exertionally mediated, and you're convincing yourself that it's catecholamine triggered. I would say don't forget about the possibility that they could be a responder to left cardiac sympathetic denervation surgery.
SAMUEL J. ASIRVATHAM: All right. Thank you very much. Thanks, Mike Thanks, Siva.
MICHAEL ACKERMAN: Thanks, everyone.
In this Heart Rhythm Webinar Series challenging case discussion, Mayo Clinic cardiology experts Samuel J. Asirvatham, M.D.,Abhishek J. Deshmukh, M.B.B.S., Siva K. Mulpuru, M.D., M.P.H., Ammar M. Killu, M.B.B.S., and Christopher V. DeSimone, M.D., Ph.D., with guest panelist Michael J. Ackerman, M.D., Ph.D., discuss genetic testing and rhythm disorders.
Related Presenters






Interests Acquired and congenital long QT syndrome (LQTS) Brugada syndrome Cardiac channelopathies Catecholaminergic polymorphic ventricular tachycardia (CPVT) Hypertrophic cardiomyopathy (HCM) Molecular autopsy Molecular ...
Related Videos