Chapters
Transcript
MACEY NELSON: Good evening, and thank you for joining tonight's webinar, "Optical Surgical Treatment of Breast Reduction and Localisation," a panel discussion on advancing the standard of care and improving outcomes.
We are pleased to have Dr. Karen Barbosa, Dr. Anthony Lucci, and Dr. Joshua Mondschein leading us through the discussion. Dr. Karen Barbosa is an oncoplastic breast surgeon from Alaska Breast Care Specialists. Dr. Anthony Lucci is a breast surgeon at MD Anderson Cancer Center and a professor in the Department of Breast Surgical Oncology, University of Texas. And finally, we have Dr. Josh Mondschein. He is a radiation oncologist with Tennessee Oncology, Provision Proton Therapy Center.
Throughout tonight's presentation, you will see a pop-up banner prompting you to ask a question. Please use the button below to ask those questions. Additionally, tonight's multidisciplinary faculty will review a few different cases, sharing their approach, technique, and decision-making process for both surgery and radiation therapy.
To encourage an interactivity with all of you, our faculty will present polling questions asking you, what would you do? When prompted, please use the function to the right of your screen to answer those questions, and please don't forget, throughout tonight's presentation, at any time, you can ask a question through the button below. This presentation will be archived for future viewing on hologiceducation.com. Thank you, and let's begin tonight's presentation. Take it away, Doctor Barbosa.
KAREN BARBOSA: Hi. Thank you all for showing up. I'm happy that we could all share some social time during social distancing and the new era of COVID medicine. Hopefully, you'll get a bit of knowledge, a little bit of laughter, and a lot of education. I am happy to say that I used to practice in New York, and I relocated to Alaska.
And based on a patient's demanding me to increase my skill set, she pointed to the wall and said, look at all those degrees and diplomas. Now you have to put your big girl pants on. You're in Alaska now. [LAUGHS] So that was my introduction to taking all the oncoplastic training I had done in the past and actually putting it into use. Welcome to the world.
So I would like to get started with when I reflect on it, I think this is actually my first case that I ever did. I brought BioZorb to Alaska, and this was probably my first patient, or maybe the second patient. So let's get started and see what we got.
She's a 38-year-old young female who had a right breast invasive ductal carcinoma and DCIS. There were two lesions. Both were biopsied. The 12:00 o'clock, about a centimeter from the nipple, was about 1.7 by 1.4 centimeters. The second lesion, also biopsy and proven invasive ductal and DCIS was approximately 2.6 by 1.3 centimeters.
Both of the lesions were ER/PR positive, HER2 negative. She was a military person who had genetic testing done on base and was BRCA-negative and clinically node-negative when she presented to me. She was very concerned about fertility, and she was also very concerned about staying in Alaska.
So let's see. This is how she showed up in the office initially. And then I would like to ask you what you think about what the next step would be, after she shows up and you've got her pathology?
Do you take her to surgery for a mastectomy, take her to surgery for a lumpectomy, sentinel node? Do a bilateral MRI? Get genomic testing of the tumor upfront? And do we have poll answers?
So it'll take a bit of time to calculate that from when people answer it, so I'm just going to tell you what we did do. I actually did an MRI, because I was concerned that the two lesions might have been one large lesion. And what it showed is that it looked like on imaging that they were two discrete lesions. Axillary involvement was negative on imaging, and we elected to do neoadjuvant chemotherapy based on the size of the two tumor locations and the overall size of her breast.
So we did a post-chemotherapy MRI, and it showed that she had marked reduction in the enhancement and good clinical, good imaging response. So let's go to the next slide, and we'll show you what happened next. Based on that, we took her into surgery. I did take the two lesions out en bloc. I was concerned still, even though imaging showed they were separate, that there might have been tentacles of tumor extending between the two, and I didn't want to leave anything in her.
We took out 6, almost 7 centimeters, by 3 centimeters, by 2 centimeters. In her case, since she was one of the cases I did early on, due to the volume we resected, and to keep that fullness, I went with a spiral BioZorb. And this is probably the first time I was ever happy to get a positive margin, because it meant I could go back in.
So with two positive margins, marked chemo effect on the specimen, and one macro met node out of two nodes, I decided to go back in. And in the next image, you'll see what we took out was the spiral BioZorb in the upper-inner quadrant. She said she noticed it, and it was a little bit palpable.
So I went back in, and when we went to resect margins-- which, by the way, were negative-- I put in two low-profile BioZorbs, which I've started to use more and more. So I think it becomes a learning curve, where to use the spirals and where to use the low-profile. But I find that when I used the low-profile, and I transferred her breast tissue over the low-profile, I was still able to create ample amount of fullness, and I did it through a mastoplexy crescent technique, which I believe we can show you the post-op picture in the next slide, so that she actually looked very good post-radiation-- so much so that she came to me complaining she didn't like her other breast.
I do work by myself, and she had also discussed preoperatively that the military was going to PCS or transition her out of state, so it was very important to her that she stay in state as long as possible. So I told her to optimize her cosmetic outcome, we could wait for a year before reducing the other side and giving that a lift, which is exactly what we did.
So I did a vertical mastopexy on the contralateral side, which would raise the nipple up to the same level as the other nipple. And at the same time, that bow of tissue that we de-epithelialized in the inferior portion would then tighten up the lower pole, pushing the breast tissue up towards the upper-inner area of the breast and create that fullness that she had on the other side.
The interesting note on this case was that when I asked my plastic surgery friends how to reconstruct her, the answer always came back, put in the same size implant. [LAUGHS] When I told them they didn't have an implant, they said, well, you're in trouble. But if you look at the next slide, I think we did OK.
Well, actually, one more slide. We'll wait for it. But this slide will show you her imaging, which I think is a beautiful delineation of the BioZorb, showing the cavity. But what I really enjoy about the imaging is that you don't see any of that fibrotic scar tissue that sometimes you'll see when you're just pulling tissue and you get some seroma or scarring down, or fibrotic areas.
So I felt like it really didn't disrupt her imaging. Clinically, she felt as though everything was great. And cosmetically, she looked really good. So on her next slide, I believe-- wow, I lost all my slides. Sorry. Upper inner quadrant tumor. We planned her case prior to surgery.
And as I mentioned, she really wanted to stay in Alaska, so that gave us the latitude of waiting for all the radiation changes. If you're fortunate enough to have a plastic surgeon to work with, you can have them do the contralateral side at the same time. And a lot of times, what they'll do is they'll automatically reduce that breast a little bit further, anticipating radiation changes. And of course, we'll get Dr. Mondschein involved in that, because he's going to speak to this case in a couple of minutes.
But we did have one positive margin, and like I mentioned, it gave me the opportunity to go back. And when you're having that learning curve, like I said, I've now moved to using the low-profiles. With her one out of two nodes, we didn't do an axillary node dissection, and she continues yearly mammograms to this day, and she's on endocrine therapy. So this is her before, and let's go to the next slide. This is her symmetrization.
So this is actually pretty quickly post-op. This is probably about maybe two or three weeks out. So she is doing really well. There's still a little bit of swelling, and that'll settle a little bit and give her even better symmetry. She subsequently moved out of state, and keeps in touch, and always reminds me that the doctors she sees are really pleased. And I have been very happy to have that experience as one of my first cases out of the box.
So I think now that I've tortured you with my slides, I will pass to Dr. Mondschein, who is the radiation oncologist with us tonight, all dapper and dressed with great hair.
JOSHUA MONDSCHEIN: Well, thank you very much, Dr. Barbosa, for that lovely introduction. My name is Josh Mondschein. I'm a radiation oncologist with Tennessee Oncology in Nashville, Tennessee, and I see patients that at Provision Proton Therapy Center. I'm still riding high from clinic. One of my patients said I looked like I was in my 30s, and I turn 40 tomorrow. So I'll just live that high for the rest of the week.
So we'll advance to the first slide. So in summary, when this patient would come to me, this is a 38-year-old female, BRCA-negative, who received neoadjuvant therapy. In terms of surgical path, in terms of T stage, we have multiple residual foci of residual carcinoma occupying a volume of 5 centimeters. It's invasive ductal carcinoma, grade 2, with multi-focal DCIS present.
In terms of markers, she's ER/PR-positive and HER2-negative. In terms of nodal status, the right axillary lymph node was positive after neoadjuvant chemotherapy, and the margins were ultimately negative. And the pathologic staging was ypT2, ypN1a, ER/PR-positive.
So next slide. Let's see. If she were to come to you, and you're the radiation oncologist, what would be your treatment recommendation? Would you tell your surgeon to go back in and do a completion axillary dissection? Would you treat adjuvant RT to the right breast and local regional lymph nodes? Would you re-excise, followed by adjuvant RT to the right breast? Or is no radiation recommended?
So yeah, I think we'll probably get to the polling later, but I think we have very good randomized data from two phase III studies suggesting that radiation therapy alone is adequate for clinically node-negative, sentinel lymph node-positive patients. So the first trial, which I'm sure-- this is primarily a surgical audience-- is probably very much near and dear to your hearts is the landmark ACOZOG Z-0011 trial published in JAMA in 2011. Again, this was a study of 891 patients with T1 to T2 to invasive breast carcinoma, no palpable lymph nodes, with 1 to 2 positive sentinel lymph nodes.
All patients underwent lumpectomy and tangential whole breast radiation. Tangential whole breast radiation means that the whole breast was treated. Level 1 was treated, and parts of level 2 of the axillary a region was treated as well. And they were randomized to either axillary dissection versus no further treatment. Patients with gross extra-nodal extension were excluded.
So there was-- in this trial, the results were there was no statistically significant difference in either overall survival or disease-free survival. The study criticisms, low accrual of this trial. In the rad onc world, there's a lot of criticism of the trial because there was protocol noncompliance. So actually, some of these patients received comprehensive nodal RT, which is what most of us will recommend that you do, and 20% of the patients were lost to follow-up.
Next slide. But the AMAROS trial really does lend further evidence that radiation alone is adequate for patients who are just clinically node-negative and sentinel lymph node positive. Again, this was a robust trial of over 4,800 patients with T1 and T2 tumors with no palpable lymph nodes, and they were sentinel lymph node positive. Essentially no significant difference in axillary recurrence, and no difference in disease-free or overall survival. And the rate of lymphedema was higher in the surgery arm, which is why I would advocate for comprehensive nodal and breast RT in this patient.
So thank you very much for that presentation on case 1.
ANTHONY LUCCI: Josh, it's Anthony. Can I ask you a question?
JOSHUA MONDSCHEIN: Sure.
ANTHONY LUCCI: So we use the BioZorb a lot for repairing defects, especially during the segment mastectomies. And as Karen said, we like to cover the tissue over the BioZorb. But I've always wondered, can you maybe address for me, in your idea, how does the BioZorb-- does it actually help you? Do you like having it in there?
Some people have used seroma for planning. Some people have used-- we used to put clips around the margins. What do you think about us putting these in? Is it helpful? Does it help? I mean, what are your thoughts on it?
JOSHUA MONDSCHEIN: No, these are all really good questions. So when patients come to me, I see a whole spectrum. I see some patients who have surgical clips in place. I see patients with no clips in place. And sometimes, I'll see patients who have a BioZorb in place.
And so during the first phase of radiation, when we're treating breast and comprehensive nodes, for the first 50 Gray, we're going to deliver that to the whole breast and levels one, two, three, supra-clad, infra-clad regions. But during the boost phase, which is the last five treatments, where we're really trying to decrease the local regional recurrence in the surgical cavity, it's extremely important for us to know exactly where that is.
So the issue is, especially after oncoplastic surgery where tissues are rearranged, it can be very difficult to really outline the discrete borders of the cavity. So for me, I find it much, much better if a BioZorb is in place. I think a lot of people believe in clips, but that can be pretty difficult, especially when they migrate.
ANTHONY LUCCI: OK, yeah. I just wanted to get your opinion on that, since we're putting them in. And I always wanted to know what your thoughts are. Do you like having it there? Is it helpful? So--
JOSHUA MONDSCHEIN: Yeah, extremely helpful. It makes it a lot easier for the boost phase.
ANTHONY LUCCI: Good to know, OK. So I think are we moving on to case two? Here we go. So I'm Anthony Lucci. I'm one of the breast cancer surgeons at MD Anderson. When we were asked to do this, the idea was, could we come up with some cases that would really generate thought and address issues that are contemporary problems or things that require some discussion or thought?
So I want to present the second case, because I think it brings up something that not only comes up every day in clinic, but may come up even more in the future as we have issues with resource utilization, and that's the question of radiation after lumpectomy for DCIS. So this case is going to discuss a DCIS. In this case, you can see it's a 62-year-old patient with a 1.5-centimeter mammographically-detected DCIS, 100% ER-positive, 90% PR-positive, HER2-negative.
The imaging might be a little difficult to see, but you can see the circled area of calcifications here on the magnified view, and the red arrow gives it up right there. So clear, almost textbook illustration of pleomorphic calcifications consistent with DCIS confirmed by biopsy. So we can go to the next slide.
So the core needle biopsy showed at 1:00 and 2:00 on the left breast, 2 centimeters from the nipple, DCIS low nuclear grade, with comedonecrosis, however, and associated calcifications confirming a concordant biopsy. We can go to the next slide.
So we're not going to talk so much about the surgical issues, but I will say we localize this area of DCIS. You can see the sick the specimen radiograph on the left with the tumor marker clip adjacent to the calcifications. You can see the calcifications in the center of the excised specimen. There's also a surgical clip attached to the specimen.
And then, on the right, we actually do sliced-specimen radiographs. I know this practice varies from different hospitals, and if this is something somebody wants to chat about, we'd be happy to do that. But our thought is, if we can do a sliced-specimen radiograph, we can try to identify any complications that are present near the margins.
And if we see calcifications near the margins, we would then attempt to resect those while we're still in the operating room to decrease our margin re-excision rate, which I will say is, I think, as low as we can get it right now. Ours is around 10%. Because we actually will act on this if we have a positive margin. We'll re-resect that at the time.
Here, you see the calcifications are pretty well outlined, with none extending to the surgical margins. We can go to the next slide. So the final surgical pathology showed DCIS-- again, low grade, with computer comedonecrosis. The margins were widely free, more than 5 millimeters at all points. And again, the rest of it was just proliferative fibrocystic changes, fibroadenomatoid change, and the calcifications were seen within the excised specimen.
We can go to the next slide. So what do our attendees think about this case? The question here is, you have a DCIS case. Would you recommend adjuvant radiation therapy? Remember, we're talking a low-grade, less than 2 centimeters DCIS in a postmenopausal patient. Would you not recommend adjuvant radiation therapy, choice B? C, what about an assay like a DCIS oncotype?
So a genomic assay that you could use to really help establish your local regional recurrence risk and make your recommendations for radiation therapy based on that? Or maybe order a different genomic assay, Prelude or something like that. Would people have any other ideas? So please give us your thoughts on that.
So in this case, our patient was referred to radiation oncology based on a small volume DCIS less than 2.5 centimeters, low grade, with widely free margins and post-menopausal status. The patient had a discussion of radiation therapy, but the final decision between the patient and the radiation oncologist was that she would not pursue radiation therapy. Interestingly, the patient also declined any endocrine therapy. She remains with no evidence of disease at 12 months.
So the real reason for presenting this case is, Josh, I wanted to ask you, because I don't think this is an issue that's going to go away anytime soon. For DCIS treated in the United States currently, around 70% to 80% of the patients following a lumpectomy would receive radiation therapy.
So interestingly, at a time, now, where we may have waves of pandemic, we could have issues with not being able to radiate every patient at that time, what do you think things-- how do you think things will change in the future regarding access to radiation? And what about these DCIS cases? Could we potentially forgo radiation in many of them? Could we use genomic assay as a way to stratify? And I would appreciate your thoughts on that.
JOSHUA MONDSCHEIN: Got it. Yeah, so I get all the easy questions, right?
ANTHONY LUCCI: Yeah, these are the worst questions, right. The ones with no good answer.
JOSHUA MONDSCHEIN: Exactly. Well, let's pull up my first slide. So I'm going to work my way methodically through the case, and I'll let you know how I reason out DCIS cases. Usually, patients will come to me and say, my surgeon says I don't need radiation. So that's always a good place to start.
So first, we'll start the just basics. What is the rationale for giving adjuvant radiation therapy? So we all know that multiple randomized studies have shown that RT decreases the risk of in-breast tumor recurrence. And the reduction is pretty much the same across the board. It's a 50% reduction in local recurrence compared to excision alone.
We know the landmark B-17 study, a randomized study of lumpectomy only versus lumpectomy and RT. And with a 15-year follow-up, a 52% reduction in breast tumor recurrence with addition of adjuvant RT. But as we all know, there's no difference in mortality or distant recurrence.
So first, I start with the basics when I see a patient like this. And I would tell them the standard of care by the National Comprehensive Cancer Center Network is to offer adjuvant whole-breast radiation therapy. The target is the whole breast for the first phase of radiation, and then followed by a boost to the tumor bed if the patient has additional risk factors, such as high-grade disease or young age.
The fractionation that we use is was developed in Europe and Canada. It's hypo-fractionation, which I believe most should be familiar with at their institutions. It's delivered over the course of three weeks-- so typically between 15 to 16 treatments to a level of 41.4 Gray, or 40 Gray-- in that range.
Another option for low-grade DCIS is to offer accelerated partial breast irradiation, which I assume many of you are familiar with as well. ASTRO, my society, actually has suitable criteria for patients who qualify for APBI. That is age greater than or equal to 50 years of age, low to intermediate-grade disease size less than 2.5 cm, resected with negative margins. But it's important to note that in the NCCN guidelines, there is an addendum which states that if the patient and physician view the individual risk as low, some patients may be treated with excision alone, so allowing you to have dialogue and a discussion with the patient and review the data.
So next slide. So this is a representation of what your radiation oncologist and how they will plan the treatment. This is 3D-based dyssymmetry. So you can see different views of a CT scan where we're treating the right breast.
I think you can make out there's a red circle, and that is the BioZorb or which is outlining the surgical cavity. And the fancy colors that you see is a graphical representation of dose. And the areas that are red, that's the hotter dose. And then, essentially, what you're trying to do is get an even dose distribution across the entire breast.
So next slide. So what is the rationale for omitting radiation therapy? So radiation is time-consuming. It could take three to four weeks. It can be costly to the health care system as a whole. And it has acute and long-term toxicities. Our goal with DCIS would be to identify a population at low risk of recurrence.
So we can do that based on histopathologic criteria, which would be low to intermediate grade DCIS, small tumors, excised with widely negative margins. Also, age, comorbidities, and patient expectations are considered as well. And in terms of data, we have two main studies that I typically refer to in the clinic.
One is an observational study, ECOG 5194, of excision without RT in women with low to intermediate-grade DCIS or high-grade DCIS. And the results were, at 12 years, a local/regional recurrence of 14.4% in the low-to-intermediate arm and 24.6% in the high-grade arm. So I think all of us would call the high-grade arm an unacceptable rate of failure and offer radiation to that cohort.
In terms of randomized studies, we have RTOG 9804. This was a randomized trial of low-risk DCIS. 636 patients, randomized to RT or observation after surgery, with a 12.4-year median follow-up. A lower recurrence rate with the RT arm at 2.8% versus 11.4%.
Less frequent invasive recurrence, is at 1.5% versus 5.8%. But as you would expect, no difference in overall survival. So these are the numbers that I typically would quote to a patient. And depending on age and a patient's expectations, they may accept a rate of failure of around 11%.
I think you alluded to, Anthony, about multi-gene assays. So oncotope DX DCIS recurrence score, for those of you who don't order this regularly, is, essentially, utilizing a 12-gene assay to predict the 10-year risk of local recurrence and guide treatment decisions.
Prospectively analyzed in 327 patients with DCIS guys on the ECOG 5194 trial. Patients are stratified into three different risk groups, low, intermediate, or high risk. And the high risk score correlates to higher rates of local recurrence and an absolute benefit of RT.
Currently, the oncotype DCIS score is not mandated in NCCN guidelines, so it remains at the discretion of the provider. It would actually be interesting to see what percentage of providers order it. I would say that in my practice, I find it to be extremely useful for patients who are really on the fence and are having a real struggle trying to figure out which way to go. But I typically have a conversation with the patient and reassure them that, listen, this, especially with the patient that you discussed, which is low-grade, ER-positive, hopefully would get endocrine therapy, that their overall risk is low, so omitting radiation would be extremely reasonable.
As we get to the age of 70, is typically what we say is a slam dunk for omitting radiation, but there are studies ongoing looking at that 60 to 70 age group to see which women can be omitted from radiation. So that would be my-- that's my long-winded answer. But that's typically what I work through in the clinic.
ANTHONY LUCCI: So do you know what's interesting? I saw the chat results. 50% said they would have ordered an oncotype DCIS so interesting right you know also I think the DCIS we found it useful. The oncotype in-- again, I agree with you completely. Patients who aren't sure, because they have no idea what their starting point is for a local recurrence rate, so they really can't even begin to guess.
And if you find, when you get the score, it's not going to give you any predictive benefit of radiation, but at least it gives you some prognostic kind of starting point. So they can say if their starting point is 7%, well, maybe a 50% reduction isn't really worth it. But if it's 40%, it would absolutely be worth it. So I agree, it's been really useful in those cases. But in low grade, the chance of a high score is so small, that's why it wasn't really used here.
JOSHUA MONDSCHEIN: Correct, correct.
MACEY NELSON: Gentlemen, we have a question from the audience. Their question is, would brachytherapy be an option?
JOSHUA MONDSCHEIN: Yes. So it's a good question. So I think we're talking about partial breast radiation. So this patient would be considered suitable by ASTRO criteria. And as I say, there are a lot of ways to skin a cat. So you can do balloon brachytherapy. You can do, basically, photon or essentially 3D-base X-rays that you would do typically for breast cancer, so partial breast photon irradiation. So there's different ways to do it. So that would be, certainly, acceptable.
There's no-- there have been randomized studies that have, basically, taken all partial breast patients, and lumped them together, and compared them to whole breast in low-risk patients, and have found no difference in local control. And it seems to be institution-dependent. So I think there are some institutions that have a lot of experience with brachytherapy and are really excellent at delivering it.
I think when we look globally, especially in systems like in Europe and Canada where not everyone has access to brachytherapy, we're seeing a move to more hypofractionated or X-ray-based treatments than brachytherapy. But 100%, brachytherapy is an option, I would say, at a well-trained center with appropriate expertise.
MACEY NELSON: Thank you.
ANTHONY LUCCI: Thank you, Josh. That really helped to answer the question. And I think it was a really good discussion of something that isn't going to go away anytime soon, the whole question of DCIS and radiate or not. So--
KAREN BARBOSA: Yeah, and I think COVID, in this time, is going to help determine and gather data. I think a lot of people are looking at trying to do less, especially in this current pandemic situation where patients are afraid to show up. So I've got an international loop of studies, and one of which is talking about looking at all the patients who've not done radiation due to that. So I think we're going to get a lot of information through this terrible disaster.
ANTHONY LUCCI: I agree with you completely. Just like collecting data on those who are now on neoadjuvant endocrine therapy, whereas before, it wasn't so common in the United States, I think that'll provide a lot of data, too. I agree completely.
KAREN BARBOSA: Yeah.
ANTHONY LUCCI: OK, so I think we have to go on the case 3 so we stay on time here so case three we'll be a little different but again in this case, we're going to switch gears and talk about triple negative breast cancer. And I think this one is going to go over some more of the surgical issues. So this patient was a 59-year-old with a triple negative T2 clinically node-negative clinical stage breast cancer. High KI-67, greater than 90%. The tumor was 2.2 by 2 by 1.5 centimeters in the upper-outer quadrant of the left breast at 2:00 or 3:00 o'clock.
As is the case with many of our patients here, we see them in a multi-team manner. So we'll see the patient along on the same day with the medical oncologist and the radiation oncologist so we can give them a really upfront, optimal recommendation of what we would recommend.
In this case, the patient also noted that she was interested in breast conservation therapy. So the team as a whole thought that she would benefit from neoadjuvant chemotherapy, especially based on her high KI-67, triple-negative status. So that was the recommendation from the team.
So here, again, you can see imaging. The tumor marking clip is present there in the left breast. Here on the lateral view, you can see that the clip there and a little bit of the posterior aspect in the upper-outer portion of the left breast. We can go to the next one.
So the patient received dose-dense AC times 3, followed by taxane. She had an excellent response, based on volumetric reduction on the repeat ultrasound. And so the plan was to surgically proceed for breast conservation therapy and a local oncoplastic reconstruction prior to her going on to whole-breast radiation therapy.
So we can go on to the next. So here, we're going to look, now, at a video that's going to be going through real quickly how we are using the BioZorb to optimally reconstruct and mark the cavity. So here's the incision being made in the upper outer-quadrant of the left breast. We're dissecting down. And as you'll see here in a minute, when we can really identify where the tumor is, and here's the segmental mastectomy specimen-- about 6, by 4, by 3.5. You can see the purple ink we used to delineate the area where the-- so we used a marking pen, just a marking pen to note where the tumor was. And so that way, we can put the BioZorb right where the tumor was.
And so now we're using the sizers to try to identify the optimal size. We always try to go, maybe, one size smaller, because we don't want palpable BioZorb. That's never a good thing. So we actually decided to go one size smaller, and we went with the low-profile, the 2 by 3 by 1 BioZorb, which actually gave us volume and allowed us to hold the tissue pillars together, sort of like a trampoline effect, without being palpable. So now we're fastening it at four sides with sutures of 4-0 prolene. We use a 4-0 prolene, the long double-armed one [INAUDIBLE]. And we actually cut off one needle and use the long suture. And then we use this to fix it in place. And then we start creating tissue flaps in the remaining tissue pillar.
So this is the adjacent tissue transfer. So we're incising the pillars, and then we mark and note the size of the flaps. In this case, they were about 5 centimeters wide and about 4 centimeters deep. And we make these superiorly and inferiorly, and then we repeat the process medially and laterally to incise the remaining tissue pillars and then be able to bring this tissue over the top of the BioZorb. And this has really been crucial in replacing the volume, but not having a palpable BioZorb that drives the patient crazy. And in fact, I can tell you, using this technique, we haven't had anyone coming in telling us that it was bothering them.
So here we go, making the opposite-side flaps. And what we're going to do is stack the flaps on top of each other. So we'll do the superior-inferior ones, re-approximate them first, and then we'll do the mediolaterals over the top of that.
So here you see the flaps being brought together. They completely cover the BioZorb at all points. There's no dimpling of the breast, because we adequately undermined and incised the tissue.
So now we're going to re-approximate the first set of flaps. Again, we're going to use a 4-0 prolene, because this keeps everything together. It doesn't fall apart. And no, we have not had any issues with infection. We've done literally hundreds of these with no problem.
And so here we are, re-approximating the first set of flaps. You can see the BioZorb is now completely covered in the spot where the tumor sat previously. And so we're going to pull these flaps together. And once these are together, we'll go to the opposite set of flaps, which are going to sit on top of those kind of like a stack of coins or pancakes, whatever you want to call it, and fill in the volume of the defect.
So you'll see it as we pull up and pull these flaps together. And again, you don't want to dimple the skin of the breast. So you don't even have to tie the sutures too tightly together. You can even lead them a little bit loose, like a lattice effect, because those little lattices will still support the skin and keep it from dimpling downward.
Here we go with the other set of flaps, going in the other direction. And so our flaps are now being re-approximated in the opposite direction over the top of the other flaps. And again, same suture. And again, we don't necessarily tighten it all the way. I think you'll see here, you don't have to pull it together so you dimple the skin. You can actually leave them almost like a scaffold or a lattice.
And you'll see here, the suture is not tied all the way together, but that little lattice is acting almost like the slats on a lawn chair, keeping everything from falling down. So there you go. You see, you don't have to tighten it completely. And I think that's an important point, because what you don't want dimpling of the skin of the breast elsewhere. You're creating a problem trying to fix this problem.
So again, you'll see, we're going to air knot it and leave the lattice in place. And now, you can push down on that. You could push really hard, and you're not going to be able to dimple that tissue. You've got your BioZorb marking the cavity for the radiation oncologist and taking up some volume. You're now covering it with your flaps that are going to replace that volume.
And you'll see in the end here, we just close in the standard way, 3-0 vicryl interrupted and a 4-0 running monocryl suture. This was 20 days post-op. You can see almost no defect. And by the way, this was a single incision, because the patient's sentinel node was just a few centimeters lateral. Here's a lateral view.
So now you're seeing the era of breast cancer treated with a single incision. Here's five weeks after radiation therapy, almost invisible. This patient has now come back, now almost 12 months after, and no defect. So obviously, very happy.
And I think this is really where we need to go with breast cancer. These early, small breast cancers, we can do minimal intervention, minimal incisions, get the sentinel node out, get the tumor out, and she ends up with just a tiny incision that's barely, barely visible, and with the same outcome as if we had done a mastectomy. So I think this was really-- I thought that's why I wanted to present this case.
So the final pathology showed about 20% residual cellularity. Obviously, there was chemotherapy effect. This extended over an area about 15 millimeters by 8 millimeters. The margins were widely free, and the sentinel nodes were negative.
So again, I thought this was a good one, because it's really nice. When you have these upper-outer quadrant tumors, you can potentially take both out through a single incision. And then, with the BioZorb, this low-profile BioZorb, we were able to get excellent cosmesis, even after radiation therapy, with no dimpling of the defect. And the patient was, obviously, very happy with this outcome and just told me, oh, people don't know I had breast cancer because they can't see the scar. So I think that's really, really great for everyone involved.
So we'll go to the next slide, and this is going to be just a kind of a question to see what everybody is doing out there. And we really want to know, what localization techniques do you currently use for your patients with non-palpable tumors who are going to go for breast-conserving surgery?
So A would be the good old localization wire, placed the morning of surgery, usually, with a coffee cup taped over it. We still have people that use that. Still a viable option. MagSeed, which is, again, a magnetic signal detected by the CentiMag. It's another product. Radioactive I-125 seeds. We just stopped that program because everyone's moved away from it, because of, obviously, logistical issues. If a seed gets lost, it creates a huge problem.
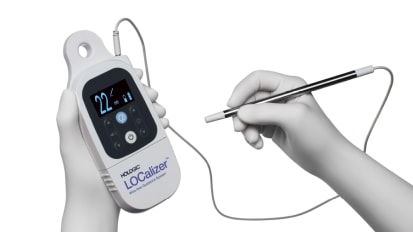
The Hologic LOCalizer, which we're going to talk about in a minute, because we've actually been using this, and it has been really, really nice. And that's actually my product of choice now. Savi Scout, which is another one. And the last choice would be other, or none. So please let us know which one you currently use. This is really more for just informational.
We can go to the-- oh, there we go. So it looks like the LOCalizer is very popular. I like that. So we go to the next slide. And this is why we like it. I mean, I have to stay, I've actually tried every single one of the items that we had on that list. Every one of them we've had and we've trialed and used. I really like the LOCalizer.
This is one of the reasons that I'm talking about this now, is I found this to be so much easier to use because of the size. It's literally-- you can see on the right is the CentiMag, the MagSeed probe, which works great. But aesthetically, it's so much nicer to have the small pencil-sized probe than this very large probe.
You can actually keep the LOCalizer in your hand while you're dissecting, because it's literally like having a pencil. So I usually keep it in one finger between a set of fingers while I use that to retract the tumor, use the cautery, and you can just flip it right back in. You can't do that with a bigger probe.
The other thing I like about it is you have unique RFID codes. So we've used it on some bracketed lesions where we had a multi-focal tumors in the breast, and we can actually Mark each one with a separate unique RFID code tag to tell which tumor is which. That has actually been really helpful as well.
The other thing that I think is really an important issue now is that we were told we had to sterilize our probe cords prior to putting the sterile drape on them. And so we've had failure with some of the probes that need to be sterilized just to repeated, repeated issues with them being heated, sterilized. And so some of those have failed. We don't have to do that with the LOCalizer. That's been a really big advantage.
The other thing I like about it as the LOCalizer tells you the distance from the lesion in millimeters. So as you get closer, you can really see exactly where you're going. I've found it, actually, to be highly accurate and really easy, simple to use. We can go to the next slide.
This was actually the first case that I did with it. You can see the tumor in the middle. You can see the RFID tag in there. And you can actually see a surgical clip getting in the way. But the tumor was right in the center. This case literally took 15 minutes or less to do. It was so simple. You can stick the probe right into the small incision and detect where you're going. It just made it really, really easy.
And we can go one more. This is just a short little video to show you the difference. Here's the LOCalizer. So there's the tumor. We can move it around. We can identify where the tag is. We can see the millimeters on the screen. We don't have to sterilize that probe. And here comes the big, sterilized probe end. And you can just see how much easier it is to manipulate within the cavity.
So I just wanted to show this because I've actually really liked the LOCalizer, and I know a lot of my colleagues just absolutely-- we really thought it was aesthetically the easiest and best to use. So I'm saying that because I really like it. So I'm interested, also, to know if anybody else has thoughts. And really, I'm excited, the fact that 100% said that's what they were using currently. So very interesting. Did we have any questions, Macey, about that? I don't know if we had any other questions.
MACEY NELSON: We do actually have a question. Not on LOCalizer. It's a question that's coming in based off of Josh's, rather Dr. Mondschein's last presentation. I'm happy to hold that question to the end just for the sake of time, for us to allow Josh to present on this case.
ANTHONY LUCCI: Sure, OK.
JOSHUA MONDSCHEIN: Got it. I probably spoke too much, so I dug myself a hole, and now I have all sorts of questions. So we'll get to those next. Yeah, so I think I've a couple of slides on this case to discussing the rad onc thought process for intact breast.
So in terms of what's the dose fractionation that we would deliver, for rad oncs, there was some controversy, but not anymore. This has become the standard. These are whole-breast ASTRO guidelines published in 2018 in one of our major journals. There was a major change that actually went on between the 2011 guideline and the 2018 guideline in terms of who qualified for hypofractionation or a shorter course of radiation-- which, again, was pioneered in Canada and Europe.
And the current guidelines, based on all available best evidence, any age, any stage, any chemotherapy, and the last thing is a rad onc-specific thing, where we're talking about making sure that you can achieve your planning goals. But the long and short of it is that the vast majority of women who receive whole breast radiation-- not nodal coverage, but whole breast alone-- would qualify for hypofractionation.
There are trials ongoing looking at whether you can hypofractionate the nodes, but we don't know if that's safe yet. So we're holding off. So next slide.
So what is a clinical challenge? Which I would say, sometimes, you don't know what you don't know. The clinical challenge can be outlining the surgical cavity when there really aren't even clips in place. So we've alluded to it beforehand, but the current methods that rad oncs use to target the lumpectomy cavity during the boost phase are either the clips or the seroma. But multiple studies have looked at this and have found that seromas can infiltrate into surrounding tissues, creating irregularities. They can overestimate the surgical cavity. And clips can migrate.
So you see illustrated on the right, pointing to different areas. If you took three different radiation oncologist, you probably have three different contours or outlines of what they think the lumpectomy cavity is. And why does that matter? Well, if you overestimate the cavity, you expose more radiation to high dose. It's not the end of the world, but in an era where we're really trying to get the best cosmetic outcome for our patients, it does matter how much healthy tissue you irradiate. Next slide.
So this is an example of the boost plan, what this looks like for the radiation oncologist. d So again, I showed you earlier some slides, what it looks like to treat the whole breast. And here, you can see what it looks like to treat just the lumpectomy cavity. These are two fields called a wedge pair, where we're just treating the BioZorb or plus a margin. And if this were a partial breast irradiation, this is exactly what we'd be treating.
And personally, for me, I do not feel comfortable doing partial breasts unless either there's a BioZorb in place, or I feel very confident that clips were placed and I can outline the cavity. But truthfully, I haven't seen a situation where that's happened so, at this point, I'm only offering partial breast X-rays for patients with a BioZorb marker. And that's my presentation. [INAUDIBLE]?
KAREN BARBOSA: OK. All right, do we have time for the next one? All right, so let's get started. Case four, I love this case, because we all have this patient in our practice at some level. Might I say she's from Alaska, and she's more extreme, because they pride themselves on their individuality.
This is a very lovely 64-year-old lady who presented to my office with left breast cancer two lesions. Both were biopsied and proven to be invasive ductal carcinoma and DCIS. One was at 2:00 o'clock 6 centimeters from the nipple, and another one was at 3:00 o'clock 3 centimeters from the nipple. Both ER/PR-positive, HER2-negative and both were approximately a centimeter.
So she started off with, I want five months off because I need to go to attend my daughter's graduation, and I'm going to help her move. So I really am not interested in doing anything right now, but I wanted to come meet you. Second, we discussed standard of care is looking at lumpectomy, sentinel node biopsy, endocrine current therapy, and doing radiation therapy. She wasn't interested in, really, anything. But I make a deal with patients that at least if they can go to the different providers and get an education, at least they're making an informed decision, rather than just turning it down off the bat. And I've seen some patients go and change their mind.
So let's go to the next slide. And this is what she had. She had-- upper arrow is her lesion at 2:00 o'clock, and her other arrow, lower, is at 3:00 o'clock. But you can see she's got really large, tautic breasts, and she has very glandular breast tissue. Younger women have denser breast tissue, which is nice and easy to do reconstructions, because you can really put a stitch in there and it'll stay. When you get into more glandular or less dense breasts, sometimes reconstruction can be difficult, and you have to be really careful about the potential for fat necrosis and devascularizing the fatty breast tissue as you're moving flaps and planes.
So let's go to the next tissue. Whoops, sorry, next slide. What would you do? Take you to surgery, get an MRI, genomic testing of her tumor, genetic testing of the patient, refer her to another surgeon? Let's go to the next slide for the sake of time so we can get everything in, and we'll get the poll answers later. And so what was done? Let's see.
We did do an MRI. It did confirm two sites. Axillary and internal nodes were considered normal. She agreed to me and talked to the radiation oncologist, the medical oncologist, and we did an oncotype just to see where she was, since she was so resistant to everything. We also enforced that despite her low score-- because she felt like, I don't need anything, and that was her interpretation of the oncotype-- it was reiterated to her that the oncotype score is based off of doing endocrine therapy, which she was opposed to doing because she had vision problems and she read online that that could complicate her vision and cause problems.
So let's go to the next slide. Let's start off with, much begging was done to get her to follow up with the consults, but she eventually went, and I actually got the notes to prove it, not just a patient nodding a head. So let's go to the next slide and talk about what we had as options. Mastectomy, sentinel node biopsy, possible chemotherapy, endocrine therapy. Mastectomy, sentinel node endocrine therapy. Lumpectomy, sentinel node radiation, possible chemo, endocrine therapy. Lumpectomy, sentinel node radiation, endocrine therapy. And lastly, neoadjuvant endocrine therapy, lumpectomy, sentinel lymph node biopsy, radiation, and endocrine therapy.
Well, based on her oncotype score, we canceled out anything with the chemotherapy as not an option, which was good, because that's concomitant with how she was thinking. But unfortunately, she wasn't up for a mastectomy, and she didn't want to do radiation, and she didn't want to do endocrine therapy. Welcome to the world.
So here is a nice couple of diagrams we look at when we do these oncoplastic procedures. We like to measure sternal notch to nipple distance, the diameter of the breast, and the nipple to inframammary fold. And when we looked at her surgery, she had these very large, tautic breasts with very glandular tissue. And basically, we were going to have to do a lumpectomy that was going to basically take out about 50% of her breast. So it was going to be a difficult reconstruction. We elected to do a Benelli. So let's go to the next slide.
These are the BioZorbs. And in her case, I actually used a spiral to give her the volume, because she didn't have a lot of firm breast tissue. It was more fatty and glandular, and I was really afraid of fat necrosis.
So let's go to the next slide. This is the wagon wheel associated with the Benelli. And if we go to the next slide, you'll see that the size of the tumor we took out was quite large. It was 6 by 5 by 1.7. And as you noticed, her breasts were not very big to start with, and so we did need volume replacement, which the BioZorb provided to us.
Her margins were negative, and I wanted wide negative margins with someone who didn't want to do anything. She did have no lymph nodes evaluated despite my best efforts because she was not interested. So let's go to the last.
The next picture is the picture of her actually on the OR table the day of surgery. Next slide this is her post-op on the day of surgery. Next slide. So I like this picture for a couple of reasons. This is her six-month post-op.
She's a little bit erythematous feminist around the nipple, because she didn't wear her bra. So she had a little bit of hypertrophic scarring. And don't listen to your patients-- this is a pearl-- when they tell you, I don't care what it looks like. Because she didn't want her nipple sized when we talked about it, and now she goes, well, I wish I'd sized my nipples a little smaller.
So at this point, you can also see a little bit of lateral deviation in that breast. That is basically due to post-operative inflammation and swelling. So let's go and see what she looks like two years later. And you can see that breast falls medially. And what you can't appreciate from the picture is how incredible the breast tissue feels. At about two years, it incorporated so nicely that you really can't tell there's a BioZorb in there.
She's thrilled. She's actually NED. Let's go to the next slide. She had her surgery in-- excuse me-- actually, January of 2016. No radiation, no chemo, no endocrine, no mammograms. Please be aware that I do remind her every time to reconsider these options, but she's pretty firm and fixed in her opinions. But she has agreed to do close follow-ups with clinical breast exam and ultrasound. And like I said, I keep re-inviting her to re-evaluate her treatment options.
But patient did great. Surgeon suffered many gray hairs. She is thrilled with her reconstruction, and this is clearly a case where the surgeon couldn't do much because of the patient not electing to follow through with standard of care. But her cosmetic outcome is excellent, and at least she's in the fold. And if anything should change in the future, we might have better luck at getting her to be more proactive.
All right? Thank you.
MACEY NELSON: And we do have some questions. This question, actually, is for Dr. Mondschein, and it was following your presentation of case two. A question came in wanting to know what your opinion is of using a marker such as BioZorb for a five-day with stereo.
JOSHUA MONDSCHEIN: Yes. Oh, so you're saying-- so the question was, stereotactic body radiation therapy? Was that the question? Is--
MACEY NELSON: I believe so. That's the whole question.
JOSHUA MONDSCHEIN: So the big question there to unpack is, should we be using stereotactic body radiation therapy for breast cancer? So for those of you who aren't familiar with stereotactic, or it's SBRT or Stereotypic Body Radiation Therapy, very quickly, the general concept is you're giving a very high dose of radiation-- typically in the realm of 8 to 10 Gray or higher. For lung, we use as high as 18 Gray, but typically, you're thinking 8 to 12 Gray, and in small number of fractions to a small target. Basically, the idea is that cell kill is actually different with stereotactic radiation. You're actually directly damaging vasculature and DNA, whereas usually, X-rays work by ionizing water, creating free radicals, which indirectly damage DNA.
So the next question is, should we be giving these super high doses of radiation therapy to the breast? And those are-- that question is being looked at in randomized studies. So I would tell you that yes, if you're doing a randomized trial, then you should use a BioZorb for that trial to really ensure that you're treating the right area. Off-trial, I would not offer SBRT.
Interestingly, I recommend that everybody takes a look at the FAST-Forward study, which was just released from Europe, published in The Lancet, just hot off the presses, I believe, last month. And basically, the Europeans looked at delivering-- it's not a stereotactic dose, but it's a five-fraction regimen of a little over 5 Gray per fraction. And they compared it to the Canadian fractionation, and they found no difference in local control.
So that will not immediately become a standard of care in the United States, but it's going to be hotly debated, I'm sure, at our next meeting. And the question will be, where does that FAST-Forward regimen fit into the American fractionation scheme? But circling back to the stereotactic question, it's-- I would only offer it on trial.
MACEY NELSON: Thank you. So we have more questions. This would be for Dr. Lucci and Dr. Barbosa. Who do you believe is an ideal patient and candidate for oncoplastic techniques or reconstructive lumpectomy?
KAREN BARBOSA: I'll take it. [LAUGHS]
ANTHONY LUCCI: Karen, you go with it.
KAREN BARBOSA: What I would say is, oncoplastic surgery is a philosophy, and it's meant for everyone. It's really about optimizing cosmetic outcome, and planning your incisions and your reconstructions prior to walking into the OR. So there is no patient that's not a candidate, and it really comes down to a discussion with the patient before you take them to surgery on how did they want to look?
Are they happy with their breasts currently? Do they want to keep them looking the same as they are? Do they want something that might even be considered a little bit of an improvement? We now have the ability to do a reduction at the same time as doing a lumpectomy, and a lot of my patients really enjoy that.
And while we have the benefit of taking wider margins on a reduction and a lumpectomy being joined together, the other thing is, a lot of these women suffer neck pain and back pain, and they wouldn't get it done because they didn't want to go through the hassle of a reduction just for that, but now their cancer has prompted them. And I will tell you that those are the happiest patients.
MACEY NELSON: And what do you think are the best ways to start learning breast reconstruction?
KAREN BARBOSA: [LAUGHS] Do you want me to take this one, or Anthony, do you want it?
ANTHONY LUCCI: No, you can go ahead. The only thing I would add in-- the only thing I would add in, Karen, is that you can start with simple techniques and simple things. Like actually, for us, if we need something extensive, we have plastic surgeons. If we didn't, there's more extensive techniques.
But honestly, many of the defects can be repaired with just internal tissue transfer, and adding in a BioZorb for the volume. I mean, I've found that really large areas of the breast can be restored really well without any kind of residual concave deformity as long as you take the time to fill the volume and move the tissue pillars. You may get the breast a little smaller, and then, the other side, maybe you can-- maybe later, they want to adjust. But you can really do a good job, even if you're in a limited area of resource, by using that kind of simple technique, I've found.
KAREN BARBOSA: Yeah, I'm going to second that and say, yeah, that's exactly how you want to do it. The funny thing is, I had taken several courses. And like my patient pointed out, I had all these degrees and certificates from the courses I'd taken. But I was in New York City, and I had a really great friend of mine who was a plastic surgeon. And we'd operate together.
When I went to Alaska and started doing more and more on my own just to make it convenient for patients to get it done and didn't have the scheduling issues, I started off with the courses that I had taken. And like Dr. Lucci said, a lot of it is simple stuff that surgeons are doing anyway with just advancing tissue flaps. Then you can add to it a little crescent. And then, when you get more experienced, you can do circumferential all around the areola.
Some of the critiques with just doing a crescent is that over time, the tissue of the nipple-areolar complex is very thin, and it'll get elongated. So there are things that, as you build, will start growing on prior knowledge. There's courses being taught all over the US. The ASBS has several courses. I believe Hologic has offered a course in the past, and I hope they offer it again. And these are courses that I paid thousands of dollars to go to, but it comes back to you, because the patients are so thrilled with how they look and how they feel.
My favorite comment was a patient who said, Karen, I'm so happy with how it came out. And I said to her, well, I like to think when you get lemons, make lemonade. And she looked at me, and she said, Dr. B, I wasn't perky as a young girl. These are lemon drops. You don't get better than that.
MACEY NELSON: That's a wonderful story. So we are butting up to the end of our presentation time. I just want to personally say thank you so much to Dr. Barbosa, Dr. Mondschein, and Dr. Lucci for this engaging panel presentation. I hope our audience members feel the same way. For those of you that would like to listen in to this lecture again, the archived version of this lecture will be held on Hologiced.com. In addition, there is a full library of resources available to you. So do visit Hologiceducation.com. It has been my true pleasure hosting this evening. Enjoy, and good night.
[MUSIC PLAYING]
Originally broadcast May 14, 2020
An interactive panel discussion designed to highlight optimal breast reconstruction and localization techniques along with specimen radiography through examination of actual cases and outcomes. Participants will have an opportunity to ask questions and hear about the latest breast reconstruction methods from a multi-disciplinary team, including two surgeons and a radiation oncologist, practicing in both Academic and Community based centers.
Related Presenters

Breast Surgeon, University of Maryland Shore Regional Health

Breast Surgeon, MD Anderson Cancer Center, Department of Breast Surgical Oncology, University of Texas

Radiation Oncologist, Tennessee Oncology / Provision Proton Therapy center, Nashville, TN
Related Videos